Encelta AG
Encelta AG is a swiss-based biotech developing a universal TCR-T cell platform designed to augment CD3-targeting T cell engagers.

Technology Snapshot
Indication: Broad oncology applicability – works in tandem with T cell engagers (TCEs).
Unmet need: Many patients relapse after TCE therapy largely due to T cell dysfunction
Encelta’s Approach: “AED T cells” – allogeneic T cells designed to augment TCE therapy.
Real-World Impact: Provides a scalable and therapeutically robust way to achieve broader, more durable responses to TCEs
Encelta’s Team: Led by Dr. Edo Kapetanovic (MD, PhD), synthetic immunology expert from ETH Zürich
Lead Program: AED T cells in combination with TCEs
Delivery Route: Intravenous, allogeneic “off-the-shelf” administration
Stage: Preclinical
Planned Study: Poised for early-stage trials supporting proof-of-concept in combination with established TCEs
Current Status: Platform optimization, early manufacturing scale-up, and partnership positioning underway
Incidence: TCEs already treat thousands of patients annually; more than 100 TCEs are in development
Market Growth: The global TCE market is projected to grow from $0.81B in 2023 to $18.7B by 2032 (41.8% CAGR). Sources below.
Expansion Potential: AED T cells are compatible with any CD3-targeting TCE, opening a universal, platform-level opportunity
Incidence: TCEs already treat thousands of patients annually; more than 100 TCEs are in development
Market Growth: The global TCE market is projected to grow from $0.81B in 2023 to $18.7B by 2032 (41.8% CAGR). Sources below.
Expansion Potential: AED T cells are compatible with any CD3-targeting TCE, opening a universal, platform-level opportunity
Encelta AG is a Swiss-based biotech startup developing a first-in-class allogeneic T cell therapy platform designed to augment the clinical activity of T cell engagers (TCEs) – a rapidly growing class of cancer immunotherapies. Spun out of ETH Zürich’s Systems and Synthetic Immunology department in 2023, Encelta’s core technology, Allogeneic-Engineered-Decoupled (AED) T cells, addresses a major limitation of TCEs: their reliance on healthy T cells in the patient’s body, which are often highly compromised in late-stage disease.
AED T cells provide a safe, off-the-shelf source of healthy T cells that can be co-administered with any TCE to boost efficacy – without the risk of graft-versus-host disease. This approach is shaping up to offer a scalable and cost-effective strategy to enhance TCE performance across a broad range of indications.
The company is led by Dr. Edo Kapetanovic, MD, PhD, a physician-scientist and award-winning expert in synthetic immunology and cancer biology, who is driving a focused and clinically feasible strategy to deliver the next generation of immunotherapies to cancer patients.
T cell engagers (TCEs) are one of the most promising classes of cancer immunotherapies, but their clinical success is often limited by a fundamental biological constraint: TCEs rely entirely on the patient’s own T cells to mediate tumor cell killing.
This is a serious liability because, by the time patients receive TCEs, they have often undergone prior rounds of chemotherapy, radiation, or other treatments that significantly damage their T cells – not to mention the cancer itself can induce direct immunosuppression on the T cells.
As a result, many patients relapse after receiving TCEs, not because the target antigen is absent, but likely because their immune systems lack the functional T cells needed to mount an effective response.
It’s really bad, actually.
In one study of patients treated with the CD19-targeted TCE blinatumomab, 92% of those who relapsed did so with antigen-positive disease (Jabbour E, et al., Am J Hematol. 2018), strongly suggesting that relapse was not due to antigen escape, but rather to insufficient T cell activity.
Without enough healthy or functional T cells, even a perfectly targeted TCE can fail.
This creates a clear and addressable clinical need: how can we safely and effectively get a large population of healthy T cells into the patient to ensure TCEs can perform as intended?
This is the precise challenge Encelta’s AED T cells were built to solve.
Encelta’s answer to this problem combines a proper amount of innovation with a tried a tested approach: a novel class of engineered T cells that can be safely co-administered with any T cell engager to restore and amplify its anti-tumor activity.
These cells, called Allogeneic-Engineered-Decoupled (AED) T cells, are an off-the-shelf “TCR-T cell therapy” that have been carefully engineered to simultaneously: i) retain full expression of the CD3/TCR complex, allowing them to respond to TCEs, while ii) not inducing TCR-mediated graft-versus-host…but how?
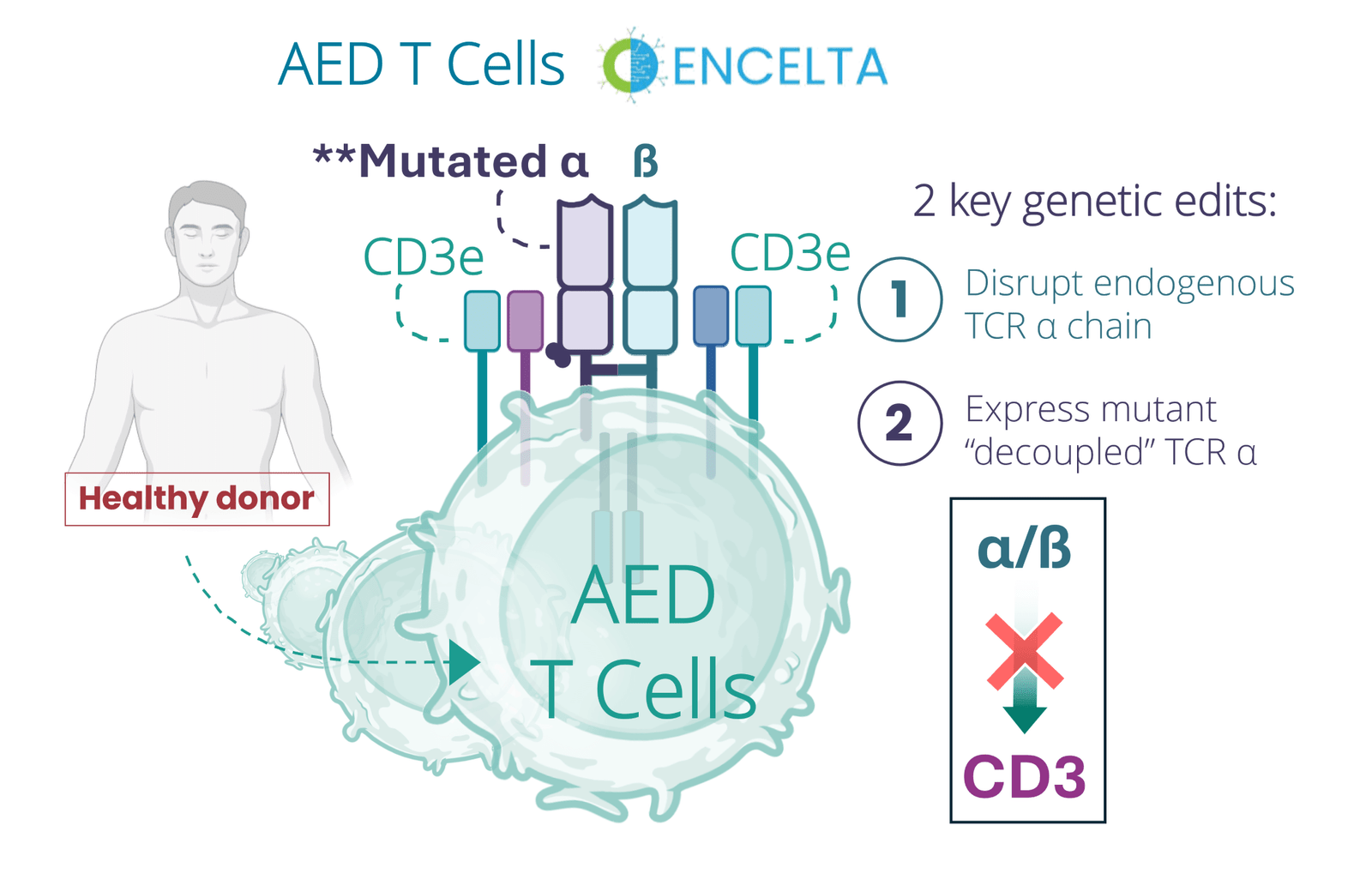
Encelta’s AED T cells incorporate two precise genetic edits:

So, the mutant TCRα is basically a decoy – its there, but its not going to do anything.
Here’s the thing though, since the full complex is still expressed on the surface of the cell, CD3 remains fully functional and capable of responding to T cell engagers. This enables AED T cells to augment the therapeutic activity of basically any TCE without risking GvHD.
So, Encelta has solved the paradox and developed a way to combine allogeneic T cells with TCEs, positioning themselves as a potential universal partner in the TCE field.
Since AED T cells can be safely paired with any TCE, regardless of the tumor target, and can be feasibly manufactured at scale, Encelta could be a highly valuable partner to the entire TCE space – and it’s a big one!
Right now, there are nine FDA-approved T cell engagers already being used to treat thousands of cancer patients across both blood cancers and solid tumors.
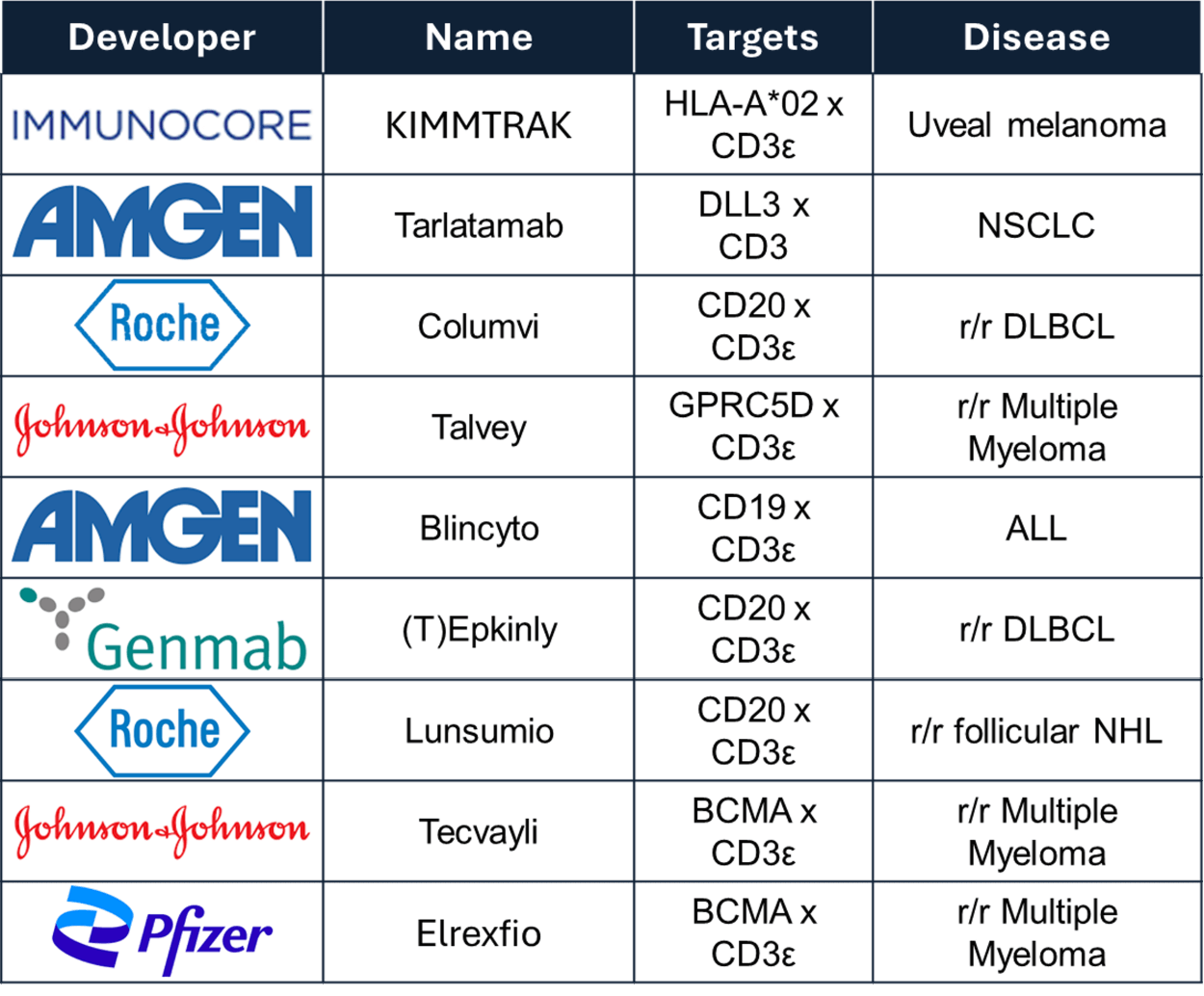
On top of that, there are over 100 more in clinical development, and many of them could benefit from a simple, off-the-shelf source of healthy T cells to boost their effectiveness.
That puts Encelta in a great position – not just to make a real difference for patients, but also to become a valuable partner to dozens of biotech and pharma companies working in the TCE space.
T cell engagers are absolutely exploding in oncology right now, and represent one of the hottest markets in healthcare. Due to AED T cells’ ability to interact with any TCE, Encelta would theoretically have access to the entire market.
According to Wise Guy Reports, the global bispecific T cell engager therapeutics market was valued at $0.81 billion in 2023 and is projected to grow to $18.7 billion by 2032, representing a remarkable compound annual growth rate (CAGR) of 41.78% from 2024 to 2032.
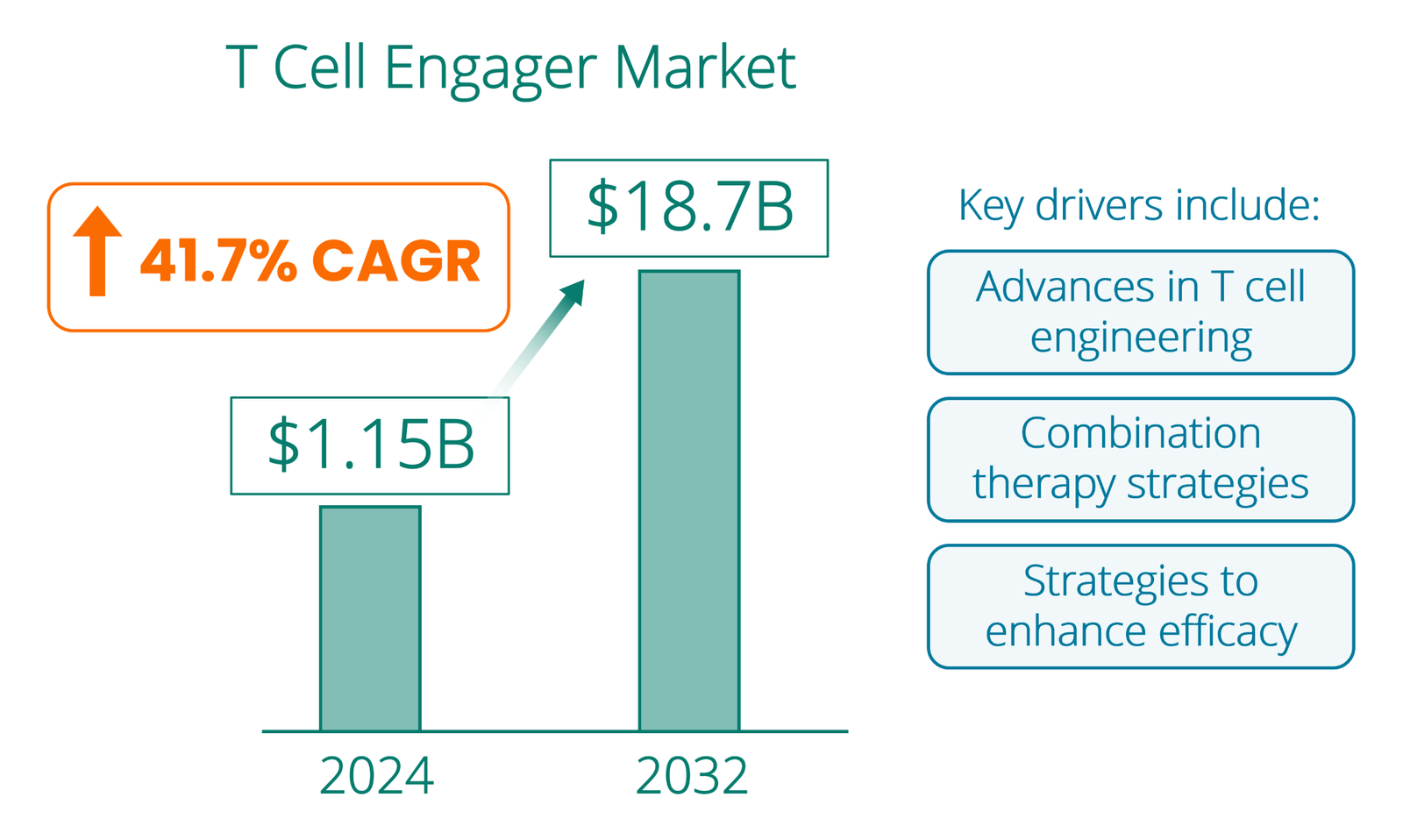
Overall, the TCE field is rapidly expanding, driven by things like advances in T cell engineering, combination therapy strategies, and strategies to enhance TCE efficacy. As TCEs become more sophisticated and broadly applied, Encelta’s AED T cells may become a highly sought after bolt-on addition to improve clinical activity.
Since AED T cells can be safely paired with any TCE, regardless of the tumor target, and can be feasibly manufactured at scale, Encelta could be a highly valuable partner to the entire TCE space – and it’s a big one!
Right now, there are nine FDA-approved T cell engagers already being used to treat thousands of cancer patients across both blood cancers and solid tumors.

On top of that, there are over 100 more in clinical development, and many of them could benefit from a simple, off-the-shelf source of healthy T cells to boost their effectiveness.
That puts Encelta in a great position – not just to make a real difference for patients, but also to become a valuable partner to dozens of biotech and pharma companies working in the TCE space.
T cell engagers are absolutely exploding in oncology right now, and represent one of the hottest markets in healthcare. Due to AED T cells’ ability to interact with any TCE, Encelta would theoretically have access to the entire market.
According to Wise Guy Reports, the global bispecific T cell engager therapeutics market was valued at $0.81 billion in 2023 and is projected to grow to $18.7 billion by 2032, representing a remarkable compound annual growth rate (CAGR) of 41.78% from 2024 to 2032.

Overall, the TCE field is rapidly expanding, driven by things like advances in T cell engineering, combination therapy strategies, and strategies to enhance TCE efficacy. As TCEs become more sophisticated and broadly applied, Encelta’s AED T cells may become a highly sought after bolt-on addition to improve clinical activity.
Related Company Profiles

Encelta AG is a swiss-based biotech developing a universal TCR-T cell platform designed to augment CD3-targeting T cell engagers.
Related Unlicensed IP
More Technology Profiles
T cell engagers for solid tumors is a subset of T cell engagers that are explicitly designed to target solid tumor indications.
Targeted alpha therapy is a cutting-edge form of radiopharmaceuticals that use alpha-emitting isotopes to deliver highly potent, localized radiation directly to cancer cells, sparing healthy
Tumor-activated therapeutics are, as the name implies, therapeutics that are designed to become active in the tumor. How is this done, what are the advantages,
More info coming soon
Share profile:
Pioneering Scientists
Key Publications

Published:
Engineered allogeneic T cells decoupling T-cell-receptor and CD3 signalling enhance the antitumour activity of bispecific antibodies
*Featured on cover! Volume 8 Issue 12
Learn how this profile was made.

Dr. Edo Kapetanovic is a medical doctor and scientist specializing in synthetic immunology and cancer biology. Kapetanovic earned his medical doctorate (MD) from the University of Rijeka in 2016 and completed his PhD in Cell and Molecular Biology at ETH Zurich in 2021 under the supervision of Prof. Sai Reddy. His doctoral research focused on engineering immune cells to overcome graft-versus-host reactions, a critical barrier to the use of donor cells in cancer therapy.
Kapetanovic’s achievements have been recognized with prestigious awards, including funding from Innosuisse and Helmut Horten Stiftung. In 2023, he was nominated for the Spark Award for his contributions to synthetic immunology. With over seven years of experience in academia and industry, he continues to drive innovation at Encelta, aiming to deliver standardized off-the-shelf cancer therapies that are accessible to more patients worldwide
Get early-stage company profiles, unlicensed tech briefs, and our analysis of rising trends in the industry delivered to your inbox.